 Image credit: Wiley
Image credit: WileyAbstract
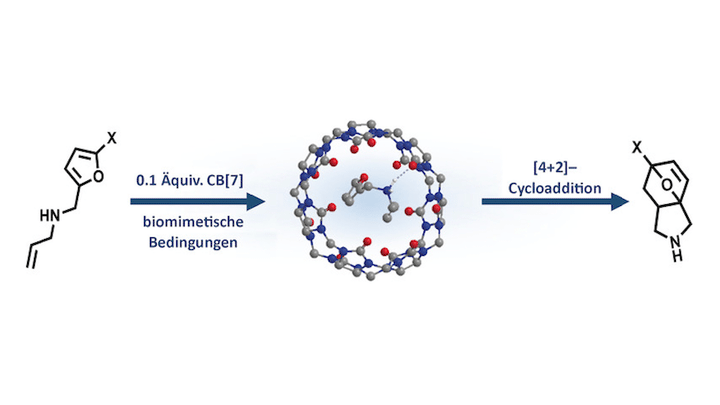
Abstract The ability to mimic the activity of natural enzymes using supramolecular constructs (artificial enzymes) is a vibrant scientific research field. Herein, we demonstrate that cucurbit[7]uril (CB[7]) can catalyse Diels–Alder reactions for a number of substituted and unreactive N-allyl-2-furfurylamines under biomimetic conditions, without the need for protecting groups, yielding powerful synthons in previously unreported mild conditions. CB[7] rearranges the substrate in a highly reactive conformation and shields it from the aqueous environment, thereby mimicking the mode of action of a natural Diels–Alderase. These findings can be directly applied to the phenomenon of product inhibition observed in natural Diels–Alderase enzymes, and pave the way toward the development of novel, supramolecular-based green catalysts.