Theoretical prediction of nanomolar and sequence-selective binding of synthetic supramolecular cucurbit[7]uril to N-terminal Leu-containing tripeptides
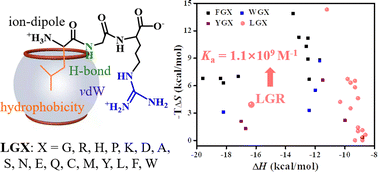 Image credit: RSC
Image credit: RSCAbstract
Molecular recognition towards peptides and proteins with high affinity by synthetic supramolecular hosts is important but challenging. In this work, we investigate the molecular recognition of the synthetic cucurbit[7]uril (CB[7]) to 17 designed N-terminal Leu-containing tripeptides in aqueous medium by molecular dynamics (MD) simulation and screen out tripeptides with high binding affinity. It is found that, compared to LGG, only the third residue is Arg (R), the binding affinity of CB[7] to LGR reaches nanomolar level with binding equilibrium constant (Ka) of 1.1x10$^9$ M$^{-1}$ . The CB[7] recognition to the N-terminal Leu-containing tripeptides is highly sequence dependent; whether changing the sequence order (from LGR to LRG) or increasing the sequence length (from LGR to LGGR), Ka decreases by about three orders of magnitude. Interestingly, substituting N-terminal Leu for its isomer Ile, the binding of CB[7] to tripeptides weakens significantly with Ka decreasing by 3~8 orders of magnitude. Thus CB[7] can effectively distinguish N-terminal Leu-containing tripeptides from N-terminal Ile-containing tripeptides. Importantly, we predict that when R is as C-terminus, regardless of N-terminal residue being of aromatic type or Leu, the binding strength is always close to the nanomolar level. Therefore, R can be introduced to rationally design novel peptides with high binding affinity to CB[7] in practical applications.