Supramolecular complexation between chain-folding poly(ester-imide)s and polycyclic aromatics: a fractal-based pattern of NMR ring-current shielding
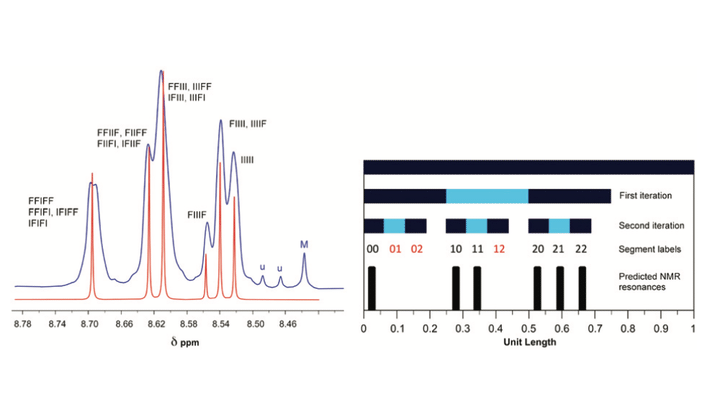 Image credit: RSC
Image credit: RSCAbstract
Polycondensation of the diimide-based diols N,N′-bis(2-hydroxyethyl)hexafluoro-isopropylidene-diphthalimide (HFDI), N,N′-bis(2-hydroxyethyl)pyromellitimide (PMDI), or N,N′-bis(2-hydroxyethyl)naphthalene-1,4,5,8-tetracarboxylic-diimide (NDI) with aliphatic diacyl chlorides ClOC(CH2)xCOCl (x = 1 to 8) affords linear poly(ester-imide)s. Homopolymers based on HFDI do not interact with polycyclic aromatics such as pyrene and perylene, as demonstrated by 1H NMR spectroscopy. However, poly(ester-imide)s containing NDI residues show significant upfield complexation shifts of the diimide resonance in the presence of pyrene and perylene, consistent with supramolecular binding of the polycyclic aromatic molecules at the diimide residues. The latter series of poly(ester-imide)s (x = 1 to 8) shows a maximum in complexation shift of the NDI resonance at x = 2. Computational simulations using a density functional based tight-binding (DFTB) method suggest that the maximum at x = 2 is due to the presence of chain folds that are geometrically optimum for a pyrene molecule to bind between pairs of adjacent NDI residues, making near-van-der-Waals contact with both diimide units. As a test of this binding model, 1H NMR studies of pyrene complexation with a copoly(ester-imide) containing both NDI and HFDI units (1 : 1 mole ratio, x = 2) were carried out. The resulting NDI resonance-pattern showed clear evidence of fractal-type character and confirmed tight chain-folding and pairwise binding of pyrene.