摘要
We demonstrate the preparation of surface‐bound cucurbit[8]uril (CB[8]) catenanes on silica nanoparticles (NPs), where CB[8] was employed as a tethered supramolecular “handcuff” to selectively capture target guest molecules. In this catenane, CB[8] was threaded onto a methyl viologen (MV) axle and immobilized onto silica NPs. The formation of CB[8] catenanes on NPs were confirmed by UV/Vis titration experiments and lithographic characterization, demonstrating a high density of CB[8] on the silica NPs surface, 0.56 nm. This CB[8] catenane system exhibits specific molecular recognition towards certain aromatic molecules such as perylene bis(diimide), naphthol and aromatic amino acids, and thus it can act as a nanoscale molecular receptor for target guests. Furthermore, we also demonstrate its use as an efficient and recyclable nano‐platform for peptide separation. By embedding magnetic NPs inside silica NPs, separation could be achieved by simply applying an external magnetic field. Moreover, the peptides captured by the catenanes could be released by reversible single‐electron reduction of MV. The entire process demonstrated high recoverability.

教授
研究方向:多组分功能组装体;非共价二聚体;超分子催化;智能软物质
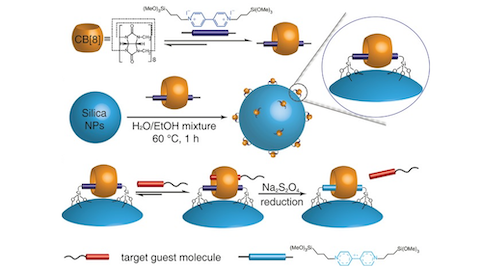 Image credit: Wiley
Image credit: Wiley