Controlling the self-assembly of cationic bolaamphiphiles: hydrotropic counteranions determine aggregated structures
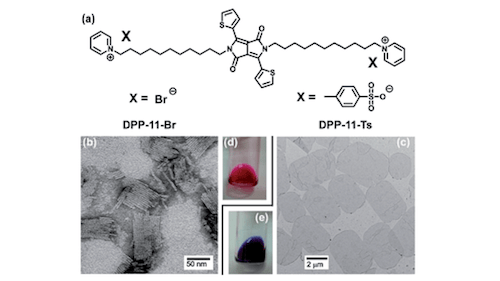 Image credit: RSC
Image credit: RSCAbstract
The role of hydrotropic counteranions in governing the self-assembled structures of cationic bolaamphiphiles is studied in this work. We reveal that the ability of a hydrotropic counteranion to induce the formation of two-dimensional (2D) planar aggregates depends weakly on its polar head, but is strongly correlated to the size and substitution pattern of its organic portion. Additionally, the shape of the obtained 2D planar aggregates can be modulated, interestingly, by both hydrotropic counteranions and embedded conjugated moieties. 2D planar aggregates with triangular, quadrangular, and hexagonal shapes are obtained, and therefore this study may provide a simple and feasible methodology for fabricating organic self-assembled structures with controlled features.